Course Code : GXPTRM-18
-
Chapter
- DEFINITION OF METHODS VALIDATION
- WHY VALIDATION ANALYTICAL PROCEDURE ?
- APPROACH OF VALIDATION
- STEPS IN METHOD VALIDATION
- SCOPE OF ANALYTICAL METHOD VALIDATION .
- TYPES OF ANALYTICAL PROCEDURES TO BE VALIDATED
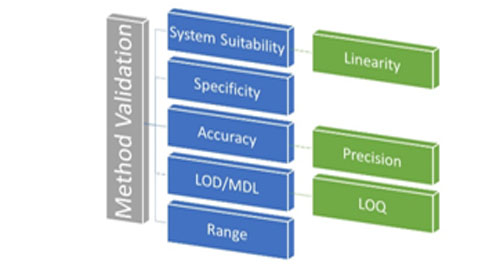
- VALIDATION CHARACTERISTICS WHICH SHOULD BE CONSIDERED ARE
- ICH, USP, AND FDA METHODS VALIDATION CHARACTERISTICS REQUIREMENTS FOR VARIOUS TYPES OF TESTS
- WRITING A TEST METHOD VALIDATION PROTOCOL
- INSTRUCTION FOR EVALUATION
- EVALUATION QUESTIONNAIRE
Course Features
- Quizzes : 7863
- Duration : 10 week
- Assessments : Self

